Switch to focused review format
What is the focused review format?
The focused review format will improve authors’ experience with Cochrane by simplifying and focusing systematic reviewing.
- Shorter reviews
- Streamlined reporting
- Technological complexities reduced
The changes create a strong foundation for Cochrane Reviews so we can innovate in how we share and use Cochrane evidence to better meet our stakeholders’ needs. Read more in Creating a focused Cochrane Review: Improvements for authors.
What are the key benefits of using the focused review format?
- Simplified reporting for authors: New templates aid authors in what to report where, facilitating adherence to expected reporting standards
- Greater impact of published Reviews: The main article focuses on the main content by moving everything else to supplementary materials, making it easier to read and use the evidence
- Better showcases the integrity of Cochrane evidence: New sections on equity, consumer involvement, and data, code and other materials
- Faster editorial processing: Shorter and more consistently reported reviews will streamline quality assurance and peer review
- Faster production processing: Shorter reviews will streamline copy editing
Should I use the focused review format in my review?
New submissions only!
If your protocol, review or update has already been submitted for editorial assessment, you do not need to switch to the focused review format. Your submission can remain in the current format through to publication.
From 21 September 2023: all new protocols, reviews and updates begun after this date in RevMan will use the focused review format; authors will be able to submit reviews using the focused review format to the Central Editorial Service.
From 31 March 2024: all new submissions of protocols, reviews and updates to the Central Editorial Service must use the focused review format.
- Already submitted, or expecting to submit your protocol or review before 30 April 2024? Continue with the current review format.
- Expecting to submit your protocol or review after 30 April 2024? Switch to the focused review format now. You will not lose any data or text in RevMan when you switch.
These timelines and guidance apply to submissions of all review types (intervention reviews, DTA reviews etc.)
Prototype review
We have a prototype review for intervention reviews using focused review format. This is a best practice example of a submission which you can use for guidance.
Best practice example prototype review
Download both the main article PDF and the zip file of Supplementary materials. Open the relevant Supplementary materials from the zip file where these are referenced in the text.
(In published reviews, in-text links will automatically open Supplementary materials in an adjacent browser tab.)
You can also create your personal copy of the prototype review as a practice review in RevMan.
Cochrane review template
If you are writing an intervention review, follow the guidance in our Cochrane review template for the focused review format, available as a practice review in RevMan for all authors. This template guides authors what to report where, ensuring the article adhere to Cochrane’s reporting standards. This will be embedded in RevMan for new intervention protocols from 21 September 2023.
How can I enable the focused review format?
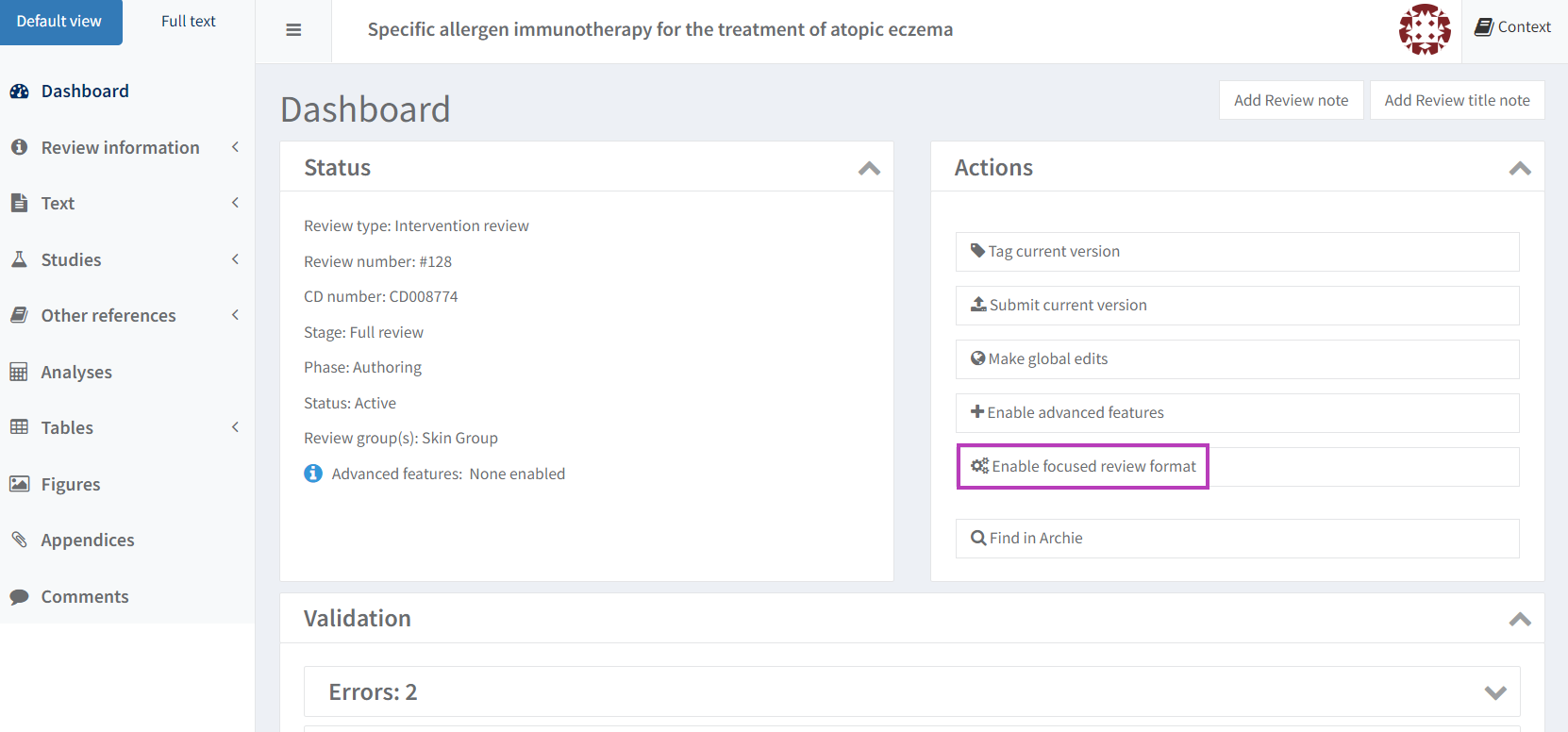
If the focused review format is enabled for a review, the Dashboard in RevMan has these changes.
To enable the focused review format, select the Enable focused review format option on the RevMan Dashboard
What checks do I need to complete if I enabled focused review format?
A fundamental change with the focused review format is that the main article includes the main content of the Review, with everything else in supplementary materials. This change makes Cochrane Reviews easier to read and share.
- What you include in the main article should support the outcomes in the Summary of findings table(s).
- Any additional data or information that supports or enhances the article should be in the supplementary materials.
The following checks are necessary for all review types:
Content checks
- Check your review is focused on a manageable scope.
- For intervention reviews, this means you should have the smallest number of comparisons to address the main objectives.
- Check you have reported your findings concisely, in plain language. Your evidence summary should be easy to read.
- Check you meet our recommended word limit of 10,000 words (see Word count)
Want to see what this means in practice? Read the prototype review.
Technical checks
Specific checks are listed in the YouTube description so you can jump to the right place in the video.
Check your supplementary materials
All appendices with search in the title will have been combined into the one ‘Search strategies supplementary material’.
Anything that does not have search in the title will become individual ‘Other supplementary materials’
Create or check your figures and tables
Figures and tables in the main article should support the outcomes in the Summary of findings table(s). Authors will need to decide which syntheses meet this criterion and add the forest plots as figures from the relevant Analysis in RevMan or create a table.
All syntheses and subgroup and sensitivity analyses will be included in the ‘Analyses’ supplementary material.
Other tables can be included as supplementary materials if they contain additional data or information that supports or enhances the article. You can list them under ‘Other supplementary materials’.
Create an 'Overview of included studies and syntheses' table
With the full ‘Characteristics of included studies’ moving to a supplementary material, authors should create an 'Overview of included studies and syntheses' table. More guidance on creating these tables is available in the Overview of Synthesis and Included Studies table (OSIS) guide and examples are included in Cochrane’s review template.
Check linking within the review
Some subheadings have changed with the focused review format. Full details are available in the Headings section.
Subheadings that were renamed will automatically update. For subheadings that are removed, the text will remain, but the linking will be lost. Broken links will be clearly highlighted within the text and authors will need to remove them throughout.
Authors should not link to Analyses within the main article text. These will not work on the published review and could be confusing for readers and should therefore be removed from the main article. These will also show as broken links within the text and authors will need to remove them throughout.
Authors should also ensure that all supplementary materials are linked from within the main article using internal links. See Supplementary materials.
Check citations within the main article
In-text citations to additional references in the main article will change from name and date (e.g. Smith 2023) to numbers in square brackets (e.g. [3]). Check no in-text citations to additional references have been embedded within a sentence as it will no longer read sensibly (e.g. [3] found that...).
Where included studies are cited, study IDs (e.g. Blair 2018) will continue to show in the text. For further details see References and citations.
All included studies must be cited at least once; either in the Results section of the main article, or in the overview of synthesis and included studies table.
If necessary, update text according to the new Cochrane review template
The focused review format uses publishing standard reporting guidelines, such as PRISMA 2020 for systematic reviews (see Reporting standards: changes to help create practical and sustainable Cochrane Reviews).
This is not a radical change, but authors should check Cochrane’s review template which details what to report where to facilitate adherence to these reporting standards. This will also guide authors in what to report in the new sections. Any sections that have been removed will automatically transfer the text to the section it’s advised that information should go. Full details about the changes to headings are available via the Headings Knowledge Base page.