Study centric data management
Have you used study-centric data and want to help us improve the process? Send in a suggestion.
All intervention reviews have study-centric data management enabled as a default system setting. See study-centric data management.
Study-centric data management does not prevent you from setting up and editing manual-input analyses in your review. See Manual-input analyses.
What is study centric data management?
Study centric data management is an innovative, time-saving approach to managing data in your review.
- Set up your synthesis criteria and analyses in RevMan in advance (at protocol stage)
- Extract data in Covidence or using our recommended templates
- Import your study data in a few clicks
- Automatically add data to populate your analyses - avoiding manual data entry errors
Study centric data management enables authors to work smarter in RevMan. Authors invest time when preparing the protocol to streamline the later analyses stages. This facilitates better defined and more focused reviews, with clearer review criteria and clearer criteria for the planned syntheses.
The first step to take advantage of study-centric data management is to complete the Review criteria section. See Differences between data management options in RevMan and Review criteria.
What are the key benefits of using study centric data management?
Managing your study data efficiently helps you to think more systematically about how to structure analyses earlier in the process.
Study results data are added in one place in RevMan and can be re-used for different analyses, including subgroup and sensitivity analyses, which saves time when generating analyses and reduces the risk of data entry errors.
Easy transfer into RevMan of included study characteristics, results data and risk of bias assessments through imports.
Easy transfer out of RevMan of included study characteristics, results data, risk of bias assessments and review analyses data through exports, for example, for further analyses in other statistical software or re-using in other Cochrane reviews.
Automatic transformation of data from arm level to contrast level data for specific analyses, where applicable. See Populate study data for an explanation of these different types of data.
Simplified interpretation of results as authors can (1) investigate broader and narrower synthesis questions based on different levels of intervention granularity and (2) subgroup and filter for sensitivity analyses based on study characteristics (covariates).
Longer term, study centric data structure will enable the introduction of new statistical methods.
Facilitates a new downloadable data package associated with published Cochrane reviews to increase impact, open opportunities for collaboration, reduce research waste, and make systematic reviewing more efficient.
Methods resources
Core methods underpinning study centric data management structure are included in chapter 2 and chapter 3 of the Cochrane Handbook for Systematic Reviews of Interventions and the Intervention Synthesis Questions (InSynQ) checklist.
Use these resources to define your criteria for including studies (PICO criteria for the review) and defining criteria for how studies will be grouped for synthesis (PICO criteria for synthesis questions).
View these webinars on How study centric data in RevMan streamlines systematic review production and Study centric data analysis and data management in RevMan: methodological background and practical application for more background information and practical demonstrations.
What are the differences between data management options in RevMan?
You can decide which data management option to use for your review. The two options are:
- Study centric data management (default): Study result data is entered and stored at the study level (results data is stored in one place, study level, and used in different analyses).
- Manual input data management (optional): Study result data is entered and stored at the analysis level. You will need to enter all data manually.
It is not currently possible to migrate all existing manual input analyses to study centric data analyses. Therefore, a review can include both study centric data analyses and manual input analyses.
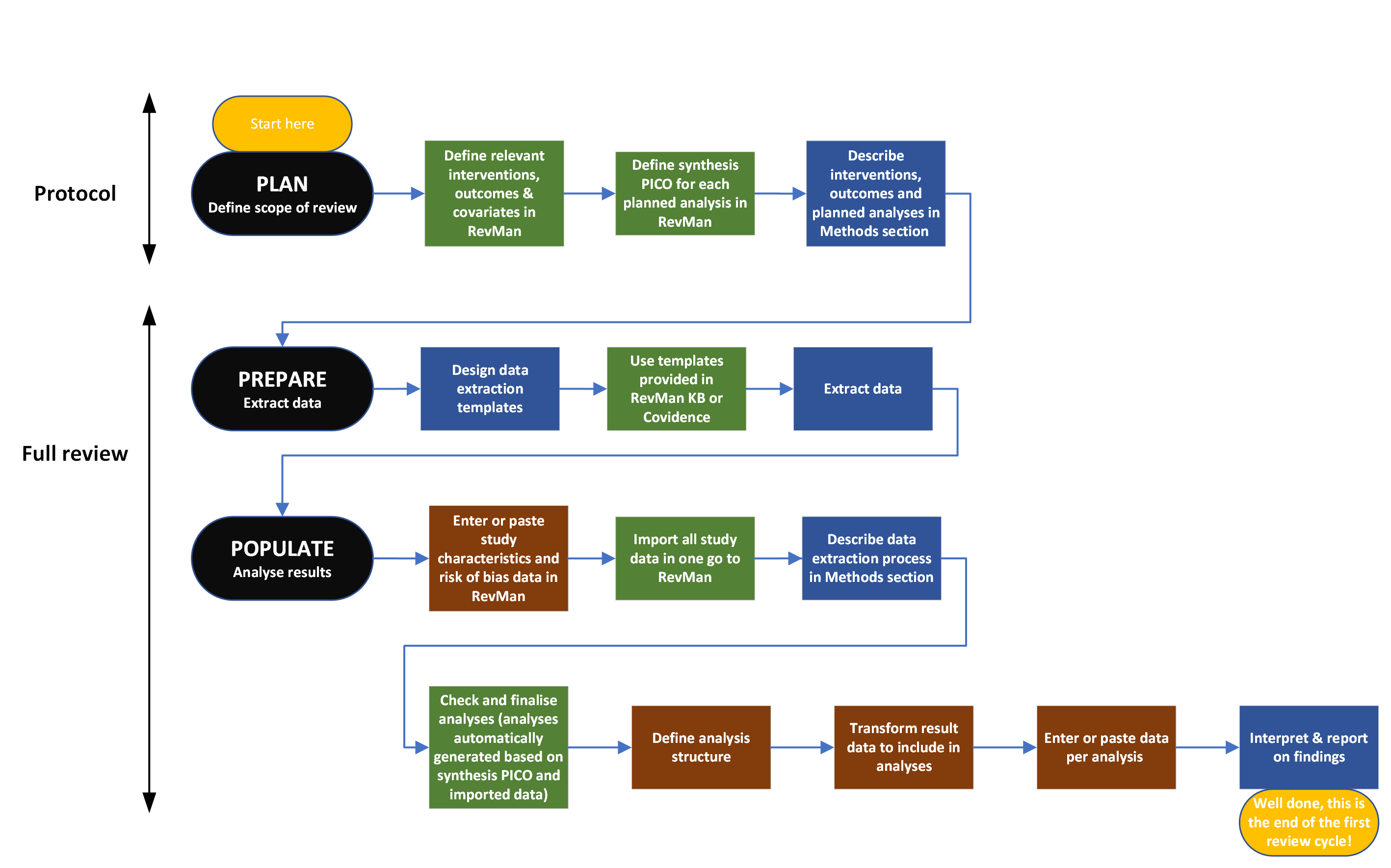
This flow diagram of the review process shows tasks specific to study centric data management (green), manual input data management (brown) and those tasks relevant to both processes (blue).
Types of analyses using manual or study data input
The table below presents the types of analyses available in two approaches and how they are set up.
Type of analyses | Manual input analyses | Study centric data analyses |
|---|---|---|
| Analyses without subgroups | Yes. | Yes. |
Subgrouping by a characteristic of the included studies | Yes. | Yes, set covariate categories for each study and choose subgroup factor in the synthesis PICO. |
| Subgroup by risk of bias | Yes. | Yes, set covariate categories for each study and choose subgroup factor in the synthesis PICO |
| Combining arms | Yes, with the help of the calculator. | Yes, done automatically. |
| Splitting control arms e.g. in analyses where the same control group is used for more than one intervention | Yes, compensate for this by "splitting" the control arm, so that participants are not double-counted. | Yes, the study will automatically be counted only once for the pooled estimate. But this is only relevant when subgrouping by intervention is implemented. |
| Create analyses with contrast data (called GIV in manual input analyses) | Yes. | Yes. |
| Create analyses with different types of granularity of interventions (e.g. any antibiotic vs placebo and A specific antibiotic vs placebo) | Yes. | Yes. |
Subgroup by most granular interventions | Yes. | Yes |
| Subgroup by variants of the outcome | Yes. | No. Use manual input analysis. |
Subgroups within studies, e.g. subgrouping the participants of a study | Yes. | No. Use manual input analysis. |