Characteristics of studies
From 3 June 2024, all intervention reviews have study-centric data management enabled as a default system setting. See Study centric data management.
Study-centric data management does not prevent you from setting up and editing manual-input analyses in your review. See Set up a manual-input analysis.
Study centric data management allows you to import study characteristics with your study data. See Populate study data.
Whether you import your study characteristics or enter them manually in RevMan, it is essential to format your study characteristics according to Cochrane's style guidelines.
This includes adding footnotes for any abbreviations mentioned in the study characteristics
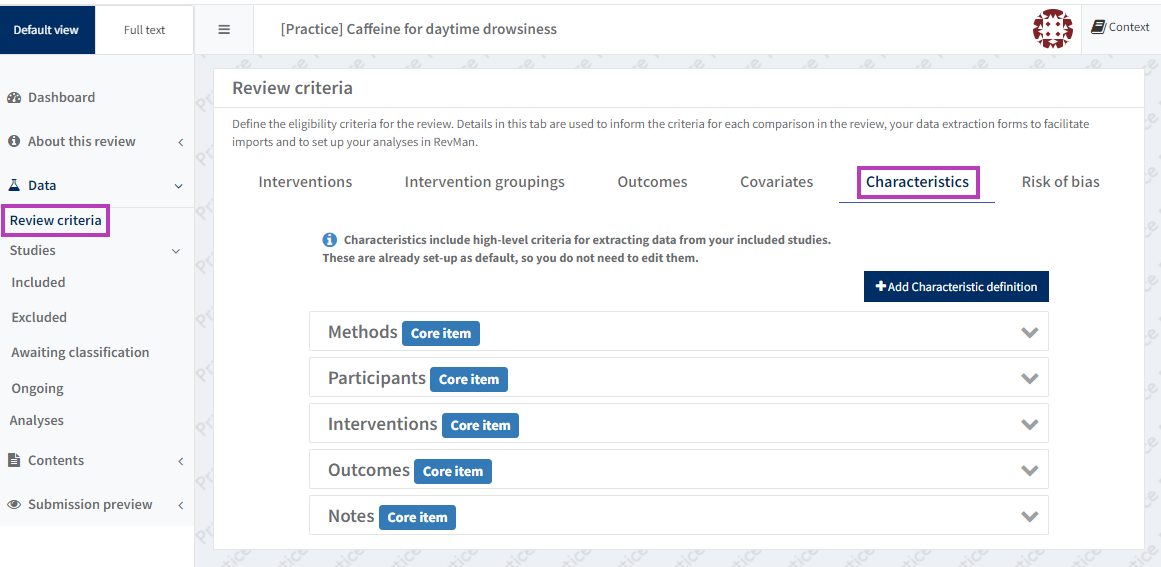
Set up characteristics of studies
The setup for the study characteristics table can be accessed via the Review criteria section.
Certain study characteristics are 'Core items' in RevMan and must be completed for each study for Cochrane reviews.
To add characteristic definitions, select the 'Add Characteristic definition' button. These characteristics will be 'User defined'.
To delete any user defined study characteristic, you can click on the bin icon that appears next to it.
Note: Characteristics of included studies tables are limited to having a maximum of 9 characteristic definitions (table headings).