Validation report
Validation errors and warnings are only relevant to Cochrane authors submitting reviews to Cochrane. RevMan subscribers can disregard validation errors and warnings.
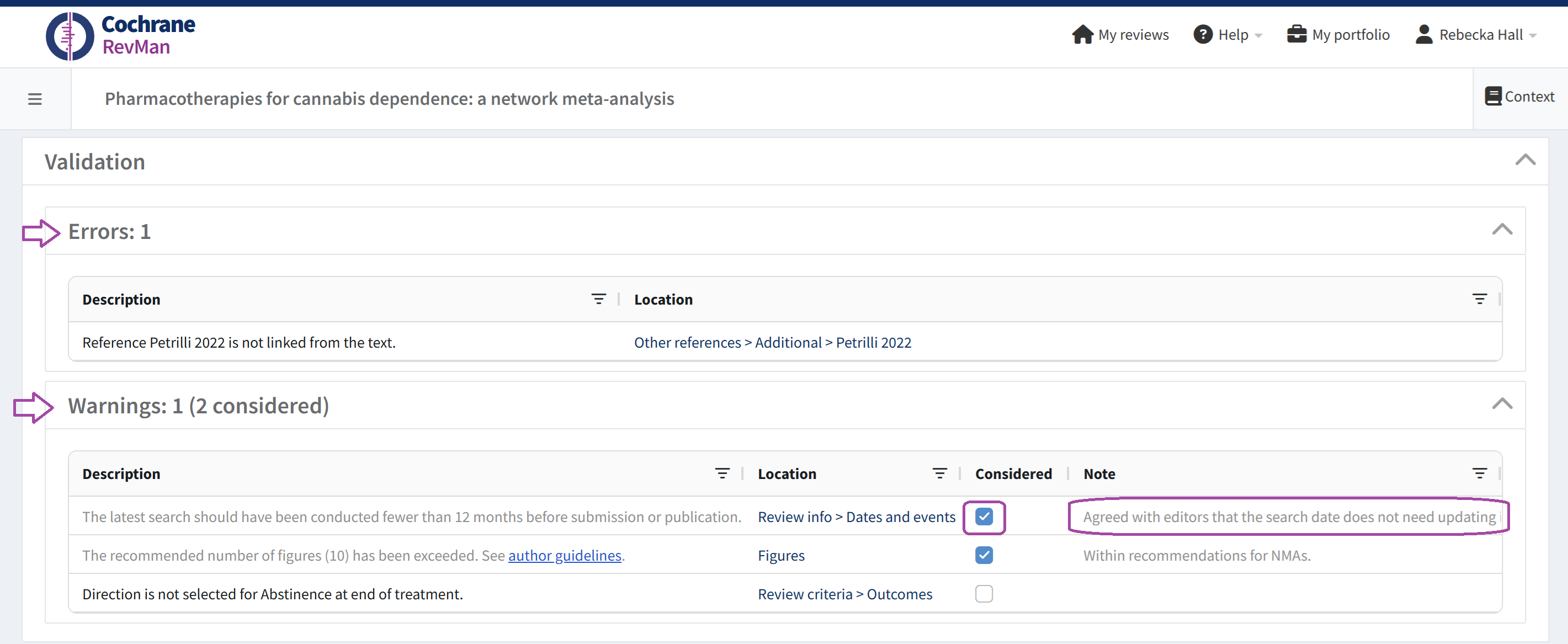
Validation report
The validation report shows you discrepancies between your review and Cochrane standards for a review.
You can see validation errors and warnings relevant to your review on the Dashboard. Expand or collapse the Validation menu to view further details.
These can be defined as Errors or Warnings. Errors MUST be addressed before submission, as you will not be able to submit your Cochrane Review to Editorial Manager with errors present.
All warnings should also be reviewed, with reference to editorial policy as required. You can mark warnings as considered and add a note describing the rationale. Always check against the relevant guidance or consult your editor before marking warnings as considered.
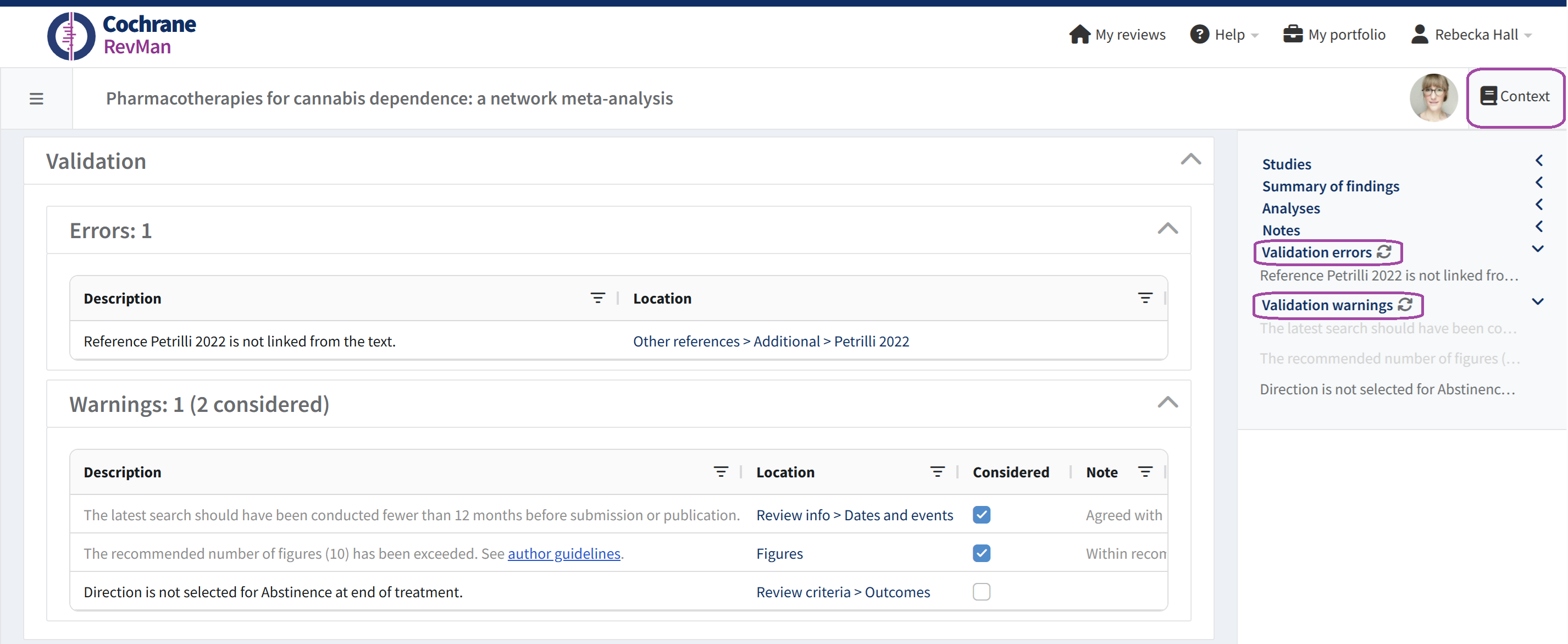
In Intervention and flexible reviews, validation errors and warnings are also visible in the right-hand Context pane. Use the links to navigate to the next issue you need to address.
Warnings and errors for empty text sections by review type
Upcoming subheading changes for Cochrane reviews
From 17 November 2025, new subheadings will be available in RevMan:
- For intervention reviews, minor changes will be made to 3 subheadings. These changes will not add or remove content to protocols or reviews, but may change the organization of the review.
- For Diagnostic Test Accuracy (DTA), methodology, overview, Quality Evidence Synthesis (QES), and prognosis reviews, larger subheading changes will be implemented. Although these subheadings are new, they reflect much of the content already in your review or protocol. For example, subheadings may be simply renamed or moved but still include the same content.
Timeline for adopting the new subheading changes:
Review Type | Editorial process* started | Protocols, reviews, and updates |
Intervention | No | Authors must use the new subheadings from 17 November 2025 . |
Yes | Authors should continue to use the old subheadings. The switch to the new subheading will happen on publication or rejection. | |
DTA, methodology, overview, QES, prognosis | No |
*All new protocols, reviews and updates will use the new subheadings from 1 May 2026 onwards. |
Yes | Authors should continue to use the old subheadings. The switch to the new subheadings will occur upon publication or rejection. |
* Editorial process is defined as manuscripts that have already been submitted to Editorial Manager, until publication or rejection; resubmissions and manuscripts in revision are also considered as having already started the editorial process.
Leaving text sections empty may result in an error or warning appearing in the RevMan validation report. An error indicates that the section cannot be left empty, whereas a warning or no validation means that the text section is optional. However, it is always recommended that you check the relevant guidance or consult your editor before marking any warning as considered.
Select the review type from the list below to view the relevant guidance on the validation rules for empty text sections:
- Intervention review type
- Diagnostic Test Accuracy (DTA) review type
- Methodology review type
- Overview review type
Please note that changing subheadings will remove all links to the text sections that have been modified. Any broken links will be clearly highlighted in the text, allowing authors to easily identify and remove or replace them. Read further information at Check linking within the review.
QES and prognosis reviews
New review templates with guidance for QES and prognosis reviews have been developed by the Qualitative and Implementation Methods Group and Prognosis Methods Group. You can now create your personal copy of the templates as a practice review in RevMan. See Review templates.
'Equity considerations’ and ‘Consumer involvement’ sections
DTA, methodology, overview, QES, and prognosis reviews will include new sections on equity considerations and consumer involvement. These sections are already available in the intervention review. When completing these sections for protocols and reviews, authors should refer to the guidance available in the intervention review template for these sections. Please note that the equity section is currently labeled 'Equity-related assessment'.