Notes: This policy covers the peer review of all Cochrane Reviews and protocols for Cochrane Reviews, including overviews, prognosis reviews and reviews of diagnostic test accuracy (DTA), and also Cochrane Editorials and supplements. The term 'Cochrane Reviews' is used to refer to both Cochrane Reviews and protocols for Cochrane Reviews. In this policy the term 'peer reviewer' describes someone who peer reviews a manuscript (previously referred to as a peer referee) and the term 'review author' refers to the author of a Cochrane Review. Further information on peer review can be found on the websites of individual Cochrane Review Groups (CRGs) and also the website of the Diagnostic Test Accuracy (DTA) editorial team.
Cochrane peer review policy statement
The Cochrane Database of Systematic Reviews (CDSR) is a peer-reviewed publication, which means that every Cochrane Review is evaluated by one or more specialists external to the Cochrane Review Group (CRG) editorial team before publication, and the Cochrane Review authors have the opportunity to revise the Cochrane Review in response to feedback. The peer reviewed status of all types of article published in the Cochrane Database of Systematic Reviews (CDSR) is outlined below.
Peer review of new Cochrane Reviews
All new Cochrane Reviews are peer reviewed before publication. CRGs are responsible for managing this process and make the final decision on whether the Cochrane Review is ready for publication.
Peer review of updated Cochrane Reviews
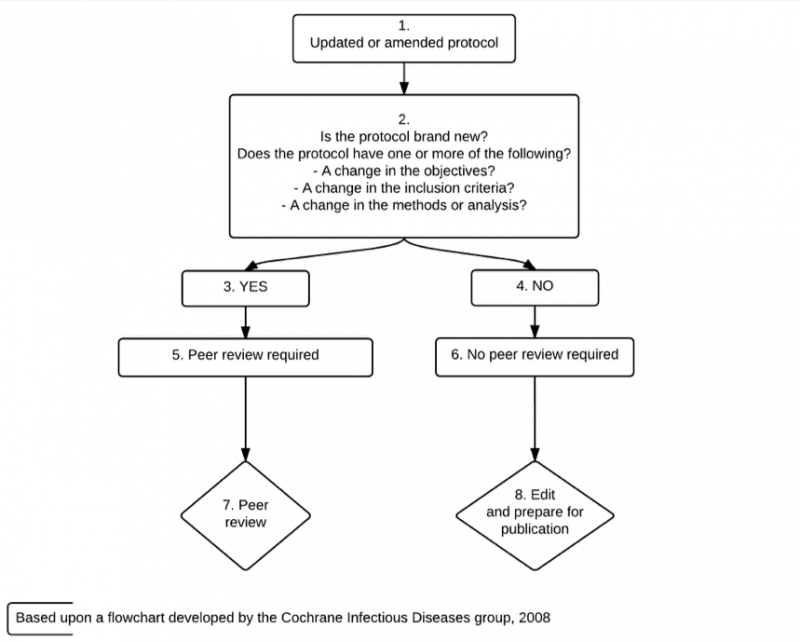
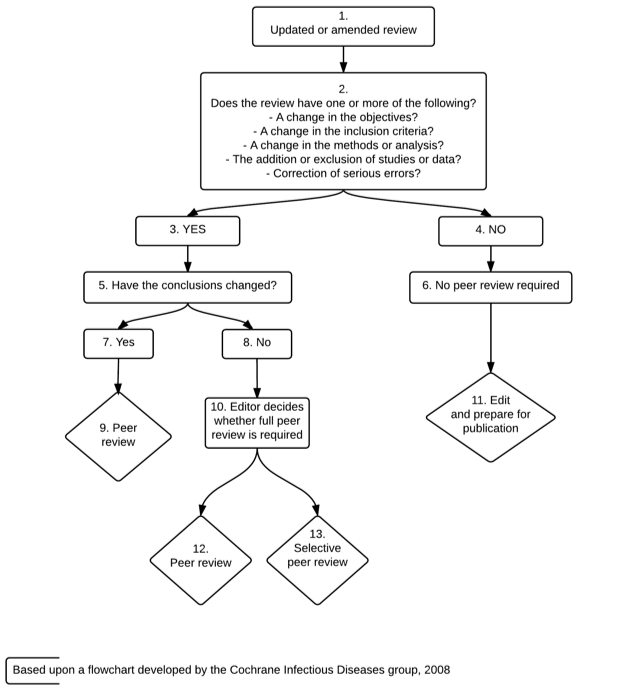
The decision as to whether an updated Cochrane Review should be peer reviewed is decided according to the circumstances outlined in Figures 1 and 2. In some cases, selective peer review will be appropriate, involving only peer reviewers with specific skills or peer review of selected parts of the review, and in other cases no peer review will be required. In all cases the updated Cochrane Review will undergo rigorous assessment by members of the CRG editorial team.
Figure 1: Decision flowchart for the peer review of updates of protocols of Cochrane Reviews
Figure 2: Decision flowchart for the peer review of updates of Cochrane Reviews
Cochrane Reviews of diagnostic test accuracy
All Cochrane Reviews of Diagnostic Test Accuracy (DTA Reviews) are peer reviewed before publication. CRGs organize peer review of the DTA Review by consumers and professional specialists; the Cochrane DTA editorial team organize methodological peer review by a methodologist, a statistician, and an information specialist with expertise in DTA review methods. All correspondence with the authors is through the CRG. All revisions must be seen and approved by the DTA editorial team. The final decision on whether the Cochrane DTA Review is ready for publication is made by both the CRG and DTA Editorial Team together; DTA Reviews only progress to publication when both the CRG and the DTA Editorial Team are in agreement.
Peer review of Cochrane editorials
Editorials may be peer reviewed, at the discretion of the Editor in Chief. The peer review process for editorials is managed by the Editorial & Methods Department. Published editorials include a 'Provenance and peer review' statement that indicates whether an editorial has been peer reviewed.
Peer review of supplements
Cochrane Database of Systematic Reviews supplements may be peer reviewed, at the discretion of the Supplement Editor and/or Editor in Chief. Supplements include a statement to indicate whether the supplement, or sections of the supplement, have been peer reviewed.
Type of peer review
From January 2019, and consistent with Cochrane’s core principles, including open and transparent communication and decision making, all CRGs will adopt a named peer review process. In a named peer review process, the Cochrane Review author and peer reviewer know each other’s names and affiliations during the peer review process.
Note that Consumer peer reviewers are exempt from the named peer review process, and may remain anonymous if they wish to do so. (Consumers still follow the policy for Declarations of potential conflicts of interest for peer reviewers).
Number and expertise of peer reviewers
As a minimum standard, every Cochrane Review will be peer-reviewed by at least one clinical/topic specialist (with a minimum of one external to the CRG editorial team) and one statistician/methodologist (who may, in some circumstances, be part of the CRG editorial team; see Number and expertise of peer reviewers).
It is expected that CRGs aim to include at least one consumer peer reviewer per Cochrane Review; the Cochrane Consumer Network has more information on this role.
Further guidance for CRGs on the number and expertise of peer reviewers, including inviting those involved in included or excluded studies to become peer reviewers, can be found in the accompanying guidance; see Number and expertise of peer reviewers.
Declarations of potential conflicts of interest for peer reviewers
Peer reviewers must declare any potential conflicts of interest every time they undertake peer review of a Cochrane Review. See Cochrane's policy on conflict of interest and Cochrane Reviews.
Further guidance on declarations of interest for peer reviewers is available; see Declarations of potential conflicts of interest for peer reviewers.
Acknowledgement and credit for peer reviewers
Peer reviewer reports are owned by the author of the report.
The names of all peer reviewers who have submitted a peer review report or completed peer review checklist during the current calendar year must be published on the CRG website, unless the peer reviewer has not consented to this (see Inviting peer reviewers). Lists from previous years must be archived and publically accessible from the CRG website. See also CRG and DTA editorial team peer review policies and procedures.
Peer reviewers should always be offered the option of acknowledgement in the Review to which they contributed.
Further options for acknowledging peer reviewers, including the format of peer reviewer acknowledgement in the published Cochrane Review, are included in Acknowledgement.
Addressing peer reviewers’ comments
Authors must provide a point-by-point response to peer review comments indicating how they have been addressed, or explaining why they have not been addressed (e.g. difference of opinion). CRGs (and the DTA Editorial Team for DTA reviews) are under no obligation to undertake any further editorial steps until they are able to check the authors have considered all questions raised by the peer reviewers.
Post-publication peer review
Post-publication peer review is available via the Comments feature (previously known as “Feedback”) present on all Cochrane Reviews (See Comments on Cochrane Reviews). All comments submitted via this channel receive a response and, if appropriate, the comment and the response from the Cochrane Review author will be published. When necessary, the review will be revised and updated in response to post-publication peer review.
Conflict resolution
Any concerns or disagreements concerning the peer review process should be resolved by the CRG. When necessary, the CRG can request that peer reviewers provide more evidence for their comments, solicit the opinion of other peer reviewers, involve the DTA Editorial team, or invite additional peer reviewers, as appropriate, to help resolve conflict. Note that authors are required to respond to peer reviewers’ comments adequately; see Ensuring that authors address Peer reviewers’ comments).
If the CRG are unable to resolve concerns or disagreements, the case may be referred to the Editor in Chief by either the authors or the CRG, using the to the Cochrane Library appeals process or complaints procedure, as appropriate.