...
If a CRG does not use the standard Cochrane peer review checklists, the following sections/statements must be included in the materials sent to the peer reviewer, and the responses reviewed and recorded appropriately in Archie.
Permissions
...
Yes
...
No
...
I am willing to be identified as the author of this peer reviewer feedback
(If no, please provide reasons below)
...
...
- I am willing to be acknowledged in the published Cochrane Protocol / Review
...
...
- : Yes or No
- I am willing to be acknowledged on the Cochrane [Review Group/DTA] website
...
...
Reasons:
...
- : Yes or NO
- If 'Yes' to either question, please state your name and affiliation as you wish it to appear:
...
...
Potential conflicts of interest
...
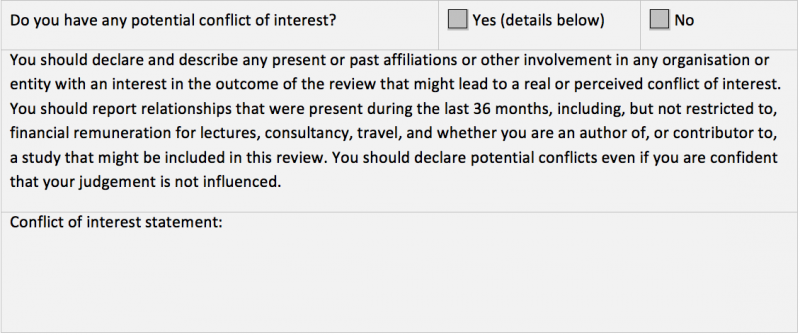
Do you have any potential conflict of interest?
...
Yes (details below)
...
No
- [insert name and affiliation]
Potential conflicts of interest
You should declare and describe any present or past affiliations or other involvement in any
...
organization or entity with an interest in the outcome of the review that might lead to a real or perceived conflict of interest. You should report relationships that were present during the last 36 months, including, but not restricted to, financial remuneration for lectures, consultancy, travel, and whether you are an author of, or contributor to, a study that might be included in this review. You should declare potential conflicts even if you are confident that your judgement is not influenced.
- Do you have any potential conflict of interest?: Yes (details below)/ No
- Conflict of interest statement:
Notes for prospective peer reviewer
Also include the following information:
- A statement that all information shared with the peer reviewer is confidential.
- A statement that peer reviewer comments may be edited for clarity and consistency with Cochrane standards.
- A statement that acceptance of the invitation to
...
- peer review for Cochrane is also taken as consent for peer reviewer details to be stored within the Cochrane peer reviewer database. (Suggestion from Ursula: We could consider updating the PR checklists to include a data protection consent option, e.g. 'By submitting this form, I consent to Cochrane storing my contact details and peer reviewer comments, for the purpose of facilitating the peer review process'?)
Managing named peer review
...
Standard Cochrane peer review checklists include a declaration of potential conflicts of interest (see Figure 1). If a CRG uses a modified version of the checklist, it must include this question, without any amendments. CRGs that do not use the standard checklists, and the DTA Editorial Team, must ensure that this question is included in the materials sent to the peer reviewer. The response from the peer reviewer should be reviewed and recorded in Archie.
Figure 1: Peer reviewer potential conflicts of interest statement
Peer reviewers must state their current affiliations(s) clearly. People with a direct financial interest in a particular intervention should not peer review a Cochrane Review of that intervention. If a peer reviewer is an author of an included/excluded trial or study (study author) this should be made clear in the Conflict of Interest statement, but they can remain a peer reviewer, as long as there is no financial interest in the intervention.
...