| Table of Contents |
|---|
Policy
1. Policy statement
...
Box 1. Definition of plagiarism
“Plagiarism is the use of others' published and unpublished ideas or words (or other intellectual property) without attribution or permission, and presenting them as new and original rather than derived from an existing source. The intent and effect of plagiarism is to mislead the reader as to the contributions of the plagiarizer. This applies whether the ideas or words are taken from abstracts, research grant applications, Institutional Review Board applications, or unpublished or published manuscripts in any publication format (print or electronic).” Source: www.wame.org/resources/publication-ethics-policies-for-medical-journals#plagiarism |
This policy relates to the Methodological Expectations of Cochrane Intervention Reviews (MECIR) reporting standard 22.
...
Table 1. Special circumstances that will generate high levels of text similarity between Cochrane Systematic Reviews, versions of Cochrane Systematic Reviews, and other articles
Special circumstance | Text similarity expected? |
Similar methods sections | Yes, Cochrane Reviews can be expected to have a high percentage of overlap in the methods section because of standardized methods. This is unlikely to cause concern unless text is copied verbatim and without correct citation |
Cochrane Review Group-specific template used for text in one or more sections | Yes, if an author uses a Cochrane Review Group template for one or more sections (e.g. background, methods), and states that a template has been used, a high percentage of overlap would be expected and should not cause concern See ‘Use of text templates’ below for details |
Protocol to review, review to update, etc. | Yes, a high percentage of overlap would be expected between certain sections of these versions (e.g. background, methods) and should not cause concern |
Generic protocol | Yes, a high percentage of overlap would be expected between certain sections of the protocol and the reviews that follow the protocol (e.g. background, methods). This should not cause concern, but it should be clear to the reader that the same text is used across a series of linked reviews |
Split and merged reviews | Yes, some overlap would be expected between the different reviews. This should not cause concern, but it should be clear to the reader that the same text is used across a series of linked reviews |
Similarities with published studies (e.g. trials described in the characteristics tables/risk of bias tables) | Yes, some overlap would be accepted here. Authors should follow the guidance (see ‘Avoiding plagiarism’) to avoid the possibility of plagiarism |
Co-publication of a Cochrane Review (including Protocol and Updates) or republication in official Cochrane journals or derivative products | Yes, a high level of overlap would be expected. This should not cause concern as long as the co-publication was agreed according to the policy |
A non-Cochrane systematic review is converted to a Cochrane Review | Yes, a high level of overlap may be expected. This should not cause concern as long as the co-publication was agreed according to the policy |
3. Avoiding plagiarism
A Cochrane Review is expected to be an original piece of academic work produced by the listed authors. Material copied from other sources may be used but should always be acknowledged. If direct quotes of more than a few words of original material are included, these should generally be indicated both by using quotation marks andby citing the source (citation alone is not enough). See examples in Table 2.
Table 2. Examples of correct citation
| Citing | The study was successful (Griffin 1990); it confirmed previous findings (Howes 1995). |
| Paraphrasing: using own words and making the source clear from the reference | It is the responsibility of systematic review authors to ensure the review conforms to Cochrane reporting guidelines including: declaring any potential conflicts of interest, that the review is free from plagiarised material and that all contributors are acknowledged (Wager 2011). |
| Using text verbatim | Wager and colleagues proposed that authors should “...ensure that contributors are properly acknowledged, that potential conflicts of interest are declared, and that the review does not contain plagiarized material” (Wager 2011). |
In Table 2, we state “It is the responsibility of systematic review authors to ensure the review conforms to Cochrane reporting guidelines including... (Wager 2011).” These are our own words, and the source is clear from the reference. If we wanted instead to use a sentence directly from the Wager paper, we would have had to do so by using quotation marks, constructing a different sentence citing the reference in brackets immediately afterwards. For example:
...
3.2. Cochrane Overviews of reviews (Cochrane Overviews)
“Cochrane Overviews of reviews (Cochrane Overviews) are Cochrane Reviews designed to compile evidence from multiple systematic reviews of interventions into one accessible and usable document” (see the Cochrane Handbook for Systematic Reviews of Intervention, Chapter 22). Authors may wish to reuse text from the original systematic reviews in a Cochrane Overview. In this circumstance, authors should follow the standard guidance to reference source material. A high percentage of overlap with other source content (e.g. a Cochrane Review) may occur, but will not cause concern if the text has been cited appropriately.
...
Table 3. Different stages in the editorial process where Similarity Check screening could occur
| Stage | Document | Recommended sections to screena |
|---|---|---|
Title | All Review Proposal Forms | All text excluding references |
Protocol | Initial submission of protocol | Backgroundb, Methodsb |
All resubmissions of revised protocols | As above | |
Substantively updated protocols (i.e. new citation version) | As above | |
Final version for publication | Screening not recommended at this stage | |
Review | Initial submission of review | Abstract, Plain language summary, Backgroundb, Methodsb, Results, Discussion, Authors’ conclusions Omit (1) matches to the published protocol from the similarity report and (2) references |
All resubmissions of revised reviews; or review ‘amendments’ | Where changes have been made to the text | |
Updates (initial version and revisions) | Abstract, Plain language summary, Backgroundb, Methodsb, Results, Discussion, Authors’ conclusions Omit (1) matches to the published protocol; (2) published previous versions of the review from the similarity reportc; and (3) references | |
Final version for publication | Screening not recommended at this stage |
a While it is possible to check an entire document for similar text, sections of a Cochrane Review, such as the methods, characteristics of studies tables, and references sections, are likely to give a high similarity score due to the nature of their content.
...
Table 4. Overview of Similarity Check process
Automatic process | Similarity Check finds and highlights overlapping text between manuscript and published material |
A similarity score is generated | |
Manual process | Similarity Check report reviewed |
Determine severity of plagiarism | |
Decide on action to be taken |
Once logged into Similarity Check, there is the option to submit different file types for screening. It is not recommended to submit the full version of the document because it may be very long and will include sections that have little value in being screened (e.g. references); see Table 3. Therefore it may be easier to select specific sections of the protocol or review to be screened. There are three possible approaches:
...
Table 5. Types of Similarity Check reports (www.ithenticate.com/training/dv-walkthrough)
Document Viewer | Default report; a detailed report that uses colour coding to compare texts, and hyperlinks to allows user to review matches. You can exclude particular sources in this mode. |
Similarity report | Displays matching sources side-by-side with sampled text. You can exclude particular sources in this mode. |
Content tracking | Enables users to see if matches were manually excluded, or if there are more than one match for the sample, and ranking of proportional match in the report. You can exclude particular sources in this mode. |
Summary report | Same information as the similarity report, but it displays matching sources above the document |
Largest matches | Ranks sample according to the word count and percentage of words that match a string of words. |
Figure 1. Example Similarity Check Document Viewer
This Similarity Check Document Viewer report shows the document being checked on the left side, highlighting matching text (in this example in red, blue and green), and the context of the matching text in the match document (Spirit MJ et al) on the right side. In this example the highlighted text in red and green match other sources than the text in blue and are not shown. The “Document Viewer” is the chosen reporting mode. Clicking on the “Text-Only Report” button will change the display to other reporting modes, which are detailed in Table 5. The “Similarity Index” applies to the entire document being checked and indicates the percentage of text from the entire document which overlaps with identifies sources (matched documents) and is shown in the upper right hand side of the report. |
4.5. Figures and images
Editorial teams should be aware that Similarity Check will not identify any plagiarized figures or images, such as line drawings and photographs. See section on figures and tables for details about copyright and identifying the copyright of figures in Cochrane Reviews.
...
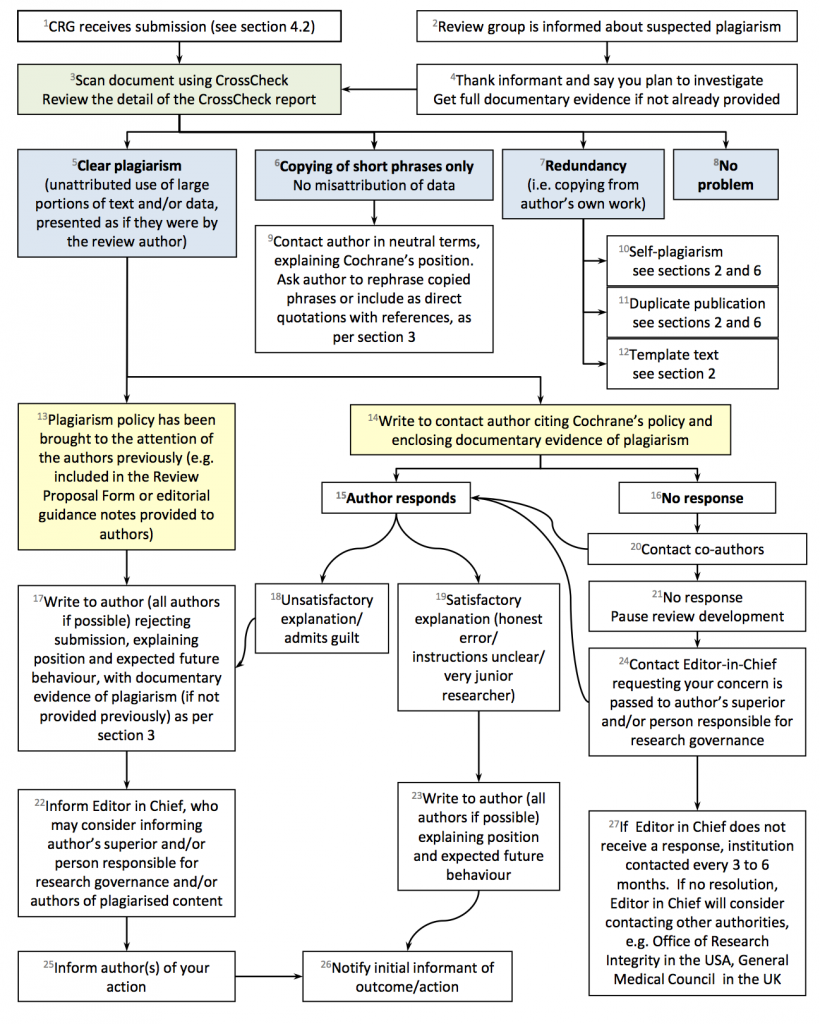
Figure 2. Flowchart: what to do if plagiarism is suspected
Adapted with permission from COPE from the flowchart: “What to do if you suspect plagiarism: Suspected plagiarism in a submitted manuscript”. |
5.1. Substantial and/or repeat instances of plagiarism
...
This applies predominantly to articles other than Cochrane Reviews. It is expected that authors of a Cochrane Review will reuse substantial parts of their protocol in the Cochrane Review that follows, for example, and this is one of the special circumstances outlined in Section 2. These special circumstances do not equate to plagiarism.
Box 2
Authors reusing text from their published works without proper attribution and/or copyright clearance may be known as duplicate publication, multiple publication, overlapping publications, redundant publication, repetitive publication, self-plagiarism, or text recycling (Wager 2014). Source: Wager E. Defining and responding to plagiarism. Learned Publishing 2014;27(1):33–42. |
The Committee on Publication Ethics (COPE) has published guidance, in the form of a flowchart, on how to deal with suspected redundant publication in a submitted manuscript. Editorial teams may wish to refer to this or discuss a particular situation with the Editor in Chief.
...
If editorial teams are alerted to suspected plagiarism in articles, including Cochrane Reviews, published in the CDSR, refer to the COPE flowchart for “Suspected plagiarism in a published article” (see publicationethics.org/resources/flowcharts) and inform the Editor in Chief. The Editor in Chief may withdraw/retract the publication as a result.
...
About this policy
The following group of people contributed to the development of this policy: Ann Jones, Anna Hobson, Gavin Stewart, Harriet MacLehose, Karin Dearness, Laura Prescott, Liz Wager, Paul Garner, Peter Tugwell, Phil Wiffen, Ruth Brassington, Sera Tort. The starting point for this policy was text drafted by Liz Wager and Phil Wiffen on publication ethics, including plagiarism.