...
Co-publication
Date | Section | What's new | |||
|---|---|---|---|---|---|
| Renamed from 'Co-publication forms and templates' and moved template information to Co-publication templates for CRG Managing Editors in the For Editors: editorial management section. | ||||
| Policy and overview
|
Policy and Overview:
Arranging co-publication of a Cochrane Review: | Updated Wiley contact info to Tony Aburrow, Senior Editor, Wiley; taburrow@wiley.com. | ||
| Updated to clarify what types of co-publication are available on both pages. | ||||
| :
Co-publication forms and templates An updated Word form has been added to ‘Co-publication forms and templates’ page. | Australian Occupational Therapy Journal changed to 'original commentary' only | |||
| Renamed from 'Co-publication forms and templates' and moved template information to Co-publication templates for CRG Managing Editors in the For Editors: editorial management section. | ||||
|
The following pages have been deleted:
| ||||
| Policy and overview | Minor updates to names/dates etc. | |||
| Agreements between journals and the CDSR for co-publication of Cochrane Reviews |
| |||
Policy and Overview:
Arranging co-publication of a Cochrane Review:
Co-publication agreements with other journals:
|
| Publishing
Co-publication forms and templates
Reuse of published Cochrane content by Cochrane Groups
The following pages have been deleted:
| |||
| Simultaneous publication: publication in a journal on the same or similar date |
| |||
| |||||
| Policy and overview | Minor updates to names/dates etc |
| Prior publication: publication in a journal before publication in the CDSR | Updated contact details. |
| Post-publication: publishing in a journal after publication in the CDSR |
| |||
| Co-publication forms |
| |||
| Agreements between journals and the CDSR for co-publication of Cochrane Reviews |
|
|
| Added Journal of Evidence-based Medicine and Chinese Journal of Evidence-based Medicine. | |
| Co-publication process: how to request and implement | Updated links and added new process diagram for clarity. | |||
| Co-publishing Updates of Cochrane Reviews | Updated links to "permission to co-publish" form and "Co-publication process". | |||
| Agreements between journals and the CDSR for co-publication of Cochrane Reviews | Co-publication agreements: added "Journal of Evidence-based Medicine" and "Chinese Journal of Evidence-based Medicine". | |||
| n/a | Updated to reflect restructure of this section. | |||
| Permission to reuse Cochrane Reviews | New section added: 'Inclusion of a Cochrane Protocol or Review in a thesis or dissertation'. | |||
| n/a | Minor copy edits. | |||
| n/a | Minor copy edits. | |||
| n/a | First publication of the co-publication policy. Policy was developed by Cochrane (Harriet MacLehose and David Tovey) and Cochrane's publisher, John Wiley & Sons, Ltd (Bryony Urquhart and Deborah Pentesco-Gilbert). |
Co-publication templates for CRG Managing Editors
...
Date
...
Section
...
What's new
...
...
Copy Edit Support
...
Date
...
Section
...
What's new
...
...
Updated contact details for Copy Edit Support and updated team membership.
Date fields in Cochrane Reviews: overview
...
Date
...
Section
...
What's new
...
...
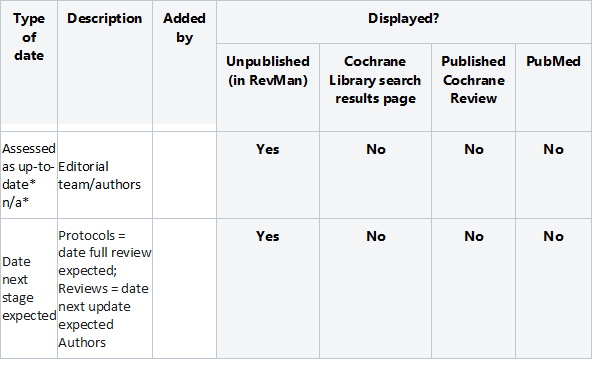
Removed ‘Assessed as up-to-date’ and ‘Date next stage expected’
Details of removed content:
...
...
...
| ||
| Publishing summaries (e.g. a ‘Cochrane Corner’) of Cochrane Reviews in another journal or resource |
|
| Simultaneous publication: publication in a journal on the same or similar date |
|
| Prior publication: publication in a journal before publication in the CDSR |
|
| Post-publication: publishing in a journal after publication in the CDSR |
|
| Co-publication forms |
|
| Agreements between journals and the CDSR for co-publication of Cochrane Reviews | Added Journal of Evidence-based Medicine and Chinese Journal of Evidence-based Medicine. |
| Co-publication process: how to request and implement | Updated links and added new process diagram for clarity. |
| Co-publishing Updates of Cochrane Reviews | Updated links to "permission to co-publish" form and "Co-publication process". |
| Agreements between journals and the CDSR for co-publication of Cochrane Reviews | Co-publication agreements: added "Journal of Evidence-based Medicine" and "Chinese Journal of Evidence-based Medicine". |
| n/a | Updated to reflect restructure of this section. |
| Permission to reuse Cochrane Reviews | New section added: 'Inclusion of a Cochrane Protocol or Review in a thesis or dissertation'. |
| n/a | Minor copy edits. |
| n/a | Minor copy edits. |
| n/a | First publication of the co-publication policy. Policy was developed by Cochrane (Harriet MacLehose and David Tovey) and Cochrane's publisher, John Wiley & Sons, Ltd (Bryony Urquhart and Deborah Pentesco-Gilbert). |
Co-publication templates for CRG Managing Editors
Defamation
...
Date
...
Section
...
What's new
...
...
Date | Section | What's new |
|---|---|---|
| General | New section that combines previous sections on editorial approval and editorial responsibility. |
...
| . |
Copy Edit Support
Date | Section | What's new |
|---|
| General |
Updated |
...
contact details for Copy Edit Support and updated team membership. |
Date fields in Cochrane Reviews: overview
Impact factor
...
Date
...
Section
...
What's new
...
...
...
| General | Removed ‘Assessed as up-to-date’ and ‘Date next stage expected’ Details of removed content: | |
| General | Updated to match current format for published Cochrane Reviews. |
| General | Revised to focus on date fields only. Title changed from "Dates and events in Cochrane Reviews" to reflect this change. |
Defamation
Date | Section | What's new |
|---|---|---|
| General |
Title chagned from "International editorial policies and good practice" to “International editorial organizations: information for Cochrane Editors”.
Highlighted where Cochrane membership opportunities.
- Updated contact details.
- Updated description of EQUATOR Network.
- Added section on ORCID (text moved from separate standalone page). Removed line about Cochrane Library publication of ORCID IDs.
Licence for publication forms
| Section changed from "Libel" to "Defamation". Updated links to publisher resources, and added section on "For Editors" plus updated contact details. |
Editorial responsibility and approval
Date | Section | What's new |
|---|
- Added new sentence regarding when contributions are owned by employer
- New page
Standard | Cochrane Review
| General | New section that combines previous sections on editorial approval and editorial responsibility. |
Editorial workflows for Cochrane Reviews
Date | Section | What's new |
|---|---|---|
| General update | Updated heading from "IMS workflow for Cochrane Reviews" to "Editorial workflows for Cochrane Reviews". Added information about using workflows for reviews with no update planned. |
EMD screening resources
Date | Section | What's new |
|---|---|---|
| Feedback from Network Associate Editors | First version published. |
Impact factor
Date | Section | What's new |
|---|---|---|
| General | 2019 impact factor added. |
| General | 2018 impact factor report added. |
International editorial organizations: information for Cochrane Editors
Date | Section | What's new |
|---|---|---|
| General |
|
| General | Updated information on activating ISMTE membership. |
Licence for publication forms
Date | Section | What's new |
|---|---|---|
|
| |
| Submitting forms on behalf of an author | Updated "may" to "must". |
| General | With effect from 25 February 2011, Managing Editors are no longer required to send paper copies of Licence for Publication forms to the Central Executive Team. |
Standard | Cochrane Review
Licence version | Release date | Explanation of changes from previous version |
| 4 |
|
|
3 |
|
Note: Note that version 3 of the Cochrane standard (green OA) licence for publication form was due to launch on 21September 2016. Due to a technical hitch the new licence for publication form was not actually available until 14 October 2016. During this time 53 Cochrane Reviews and 29 Cochrane protocols were published under the previous version of the licence for publication. We would like to apologize for this and assure all Authors affected that we will honour the new licence terms, as described above. If you have any questions about this, please contact the Cochrane Editorial & Methods Department (emd@cochrane.org). |
2 |
| Open access changes:
Other change:
|
1 | — | — (Contact the Cochrane Editorial & Methods Department (emd@cochrane.org) for information about earlier versions.) |
Standard | Protocol for a Cochrane Review
Licence version* | Release date | Explanation of changes from previous version |
| 4 |
|
|
3 |
|
Note: Note that version 3 of the Cochrane standard (green OA) licence for publication form was due to launch on 21 September 2016. Due to a technical hitch the new licence for publication form was not actually available until 14 October 2016. |
Licence version | Release date | Explanation of changes from previous version |
| 4 |
|
|
3 |
|
Note: Note that version 3 of the Cochrane standard (green OA) licence for publication form was due to launch on 21September 2016. Due to a technical hitch the new licence for publication form was not actually available until 14 October 2016. During this time 53 Cochrane Reviews and 29 Cochrane protocols were published under the previous version of the licence for publication. We would like to apologize apologise for this and assure all Authors affected that we will honour the new licence terms, as described above. If you have any questions about this, please contact the Cochrane Editorial & Methods Department (emd@cochrane.org). |
2 |
| Open access changes:
Other change:
|
1 | — | — (Contact the Cochrane Editorial & Methods Department (emd@cochrane.org) for information about earlier versions.) |
Standard | Protocol for a Cochrane Review
...
Licence version*
...
Release date
...
Explanation of changes from previous version
...
...
- External links updated.
...
3
...
...
- Field added for digital object identifier (DOI) version number.
- Revised authorship criteria for consistency with ICMJE criteria.
- Authors are directed to the Cochrane Editorial and Publishing Policy Resource for Cochrane’s updating policy.
- Removed text about Cochrane Review Group approval ahead of publication (moved to Cochrane Editorial and Publishing Policy Resource).
- Changed following author rights:
- Revised the rights to post in repositories and Scholarly Collaboration Networks (SCNs).
- Added sharing rights with colleagues.
- Updated the section on contributions owned by Employer to include US National Institutes of Health (US NIH) employees and grantees, and UK National Institute for Health Research (UK NIHR).
- Updated hyperlinks and minor text edits, including the addition of generic email addresses for all Wiley contacts and directing authors to Rightslink for permission requests.
Note: Note that version 3 of the Cochrane standard (green OA) licence for publication form was due to launch on 21 September 2016. Due to a technical hitch the new licence for publication form was not actually available until 14 October 2016. During this time 53 Cochrane Reviews and 29 Cochrane protocols were published under the previous version of the licence for publication. We would like to apologise for this and assure all Authors affected that we will honour the new licence terms, as described above. If you have any questions about this, please contact the Cochrane Editorial & Methods Department (emd@cochrane.org).
...
2
...
...
Open access changes:
- Amended to reflect the introduction of green open access.
- Introduces the new right for authors to post the final accepted version of the Protocol or Review (in a format specified by the Publisher) in an institutional repository or any repository mandated by the author’s funder, such as [insert an appropriate example], 12 months after publication.
- Introduces text to explain automatic deposition to PMC and free access on CDSR 12 months after publication.
- It also clarifies the right to post the Protocol or Review as an electronic file on the author’s own website and/or the author’s institution’s intranet (previously stated website), using the PDF version of the article available in the Cochrane Database of Systematic Reviews. These new terms are backdated to 1 February 2013 for all authors who have signed the Licence for Publication Form between 1 February 2013 and today.
Other changes:
- New sections added to the Archie and RevMan versions (were already included in the Cochrane Policy Manual version): UK Government work (Crown Copyright); US Government work; Other Government work; World Health Organization (WHO) work.
- New addition: National Institutes of Health (NIH) grantees.
- Minor editing to the introductory text in italic as shown in the Policy Manual and RevMan versions (not needed in the Archie version).
Addendum to licence for publication of a World Health Organization (WHO) manuscript as a Cochrane Review :
- Corrected the number of the addendum clauses.
- Updated the following sentence in Clause 7 to include non-WHO author rights: “The preferred format is “The World Health Organization and [Author X] and [Author Y] retain copyright and all other rights in their respective contributions to the manuscript of this Review as submitted for publication”.”
- Addendum has been prepared as a Microsoft Word (97-2003) file for authors to download from the Cochrane Policy Manual.
...
1
...
—
...
— (Contact the Cochrane Editorial & Methods Department (emd@cochrane.org) for information about earlier versions.)
Other changes:
Addendum to licence for publication of a World Health Organization (WHO) manuscript as a Cochrane Review :
| ||
1 | — | — (Contact the Cochrane Editorial & Methods Department (emd@cochrane.org) for information about earlier versions.) |
Creative Commons (Cochrane Reviews and protocols for Cochrane Reviews)
Licence version | Release date | Explanation of changes from previous version |
| 3 |
|
|
2 |
|
|
1 |
| First version; based on Creative Commons v3.0 for CC BY, CC BY-NC, and CC BY-NC-ND. |
Standard | Cochrane Editorial
...
Licence version | Release date | Explanation of changes from previous version |
| 3 |
|
|
2 |
|
|
1 |
| First version; based on Creative Commons v3.0 for CC BY, CC BY-NC, and CC BY-NC-ND. |
Standard | Cochrane Editorial
...
Licence version
...
Release date
...
Explanation of changes from previous version
...
...
...
1
...
...
New.
| Updated contact details and link for permissions. | |
| 2 |
| Updated for consistency with standard licence for a Cochrane Review, version 4. |
1 | New. |
Creative Commons | Cochrane Editorial
Licence version | Release date | Explanation of changes from previous version |
| 2 |
| Updated contact details and link for permissions. |
1 | New - based upon Creative Commons CC BY-NC licence for a Cochrane Review, version 3. |
WHO addendum | Standard licence | Protocol or Review
Addendum version | Release date | Explanation of changes from previous version |
3 |
|
|
2 |
|
|
1 |
| New |
WHO addendum | Creative Commons licences | Protocol or Review
Addendum |
Creative Commons | Cochrane Editorial
Licence version | Release date | Explanation of changes from previous version |
| 2 |
| Updated contact details and link for permissions. |
1 | New - based upon Creative Commons CC BY-NC licence for a Cochrane Review, version 3. |
...
| The WHO addendum for the Creative Commons licences for publication has been modified to permit the terms of the applicable Creative Commons 3.0 IGO Licence. This licence type grants the same re-use conditions as a standard CC-BY licence with the additional condition that when the Licensor is an intergovernmental organization (in this case the WHO), any disputes will be resolved by mediation and arbitration unless otherwise agreed. Further information on the conditions and benefits of an IGO licence can be found here. | |
1 | New. |
PAHO addendum | Standard licence | Protocol or Review
Addendum version | Release date | Explanation of changes from previous version |
32 |
|
|
2 |
|
|
1 |
| New |
WHO addendum | Creative Commons licences | Protocol or Review
...
Addendum version
...
Release date
...
Explanation of changes from previous version
...
...
1
...
...
New.
PAHO addendum | Standard licence | Protocol or Review
...
Addendum version
...
Release date
...
Explanation of changes from previous version
...
2
...
...
- Updated title to be consistent with the PAHO Creative Commons addendum.
- The ‘INSERT TITLE’ section of Clause 1 was removed.
- In Clause 1, the sentence in parenthesis referring to non-PAHO authors was removed.
- In Clause 5, a contact email address for seeking permission to re-publish was provided and the postal address for John Wiley and Sons Ltd. was removed.
- Clause 6 was split. The content of that section now forms two Clauses. Clause 7 now starts at the sentence beginning with ‘PAHO agrees that any and all copies of the Review’. All Clauses following this have been re-numbered.
...
1
...
...
New.
| ||
1 |
| New. |
PAHO addendum | Creative Commons licences | Protocol or Review
Addendum version | Release date | Explanation of changes from previous version |
1 | New. |
Managing expectations: what does Cochrane expect of authors, and what can authors expect of Cochrane?
Date | Section | What's new |
|---|---|---|
| General | Moved the linked flowchart about the editorial process for title registration to Editorial process: titles. Updated terminology and removed need for Centres to provide support with queries now covered by Cochrane Support team. |
Proposals for new Cochrane Reviews: editorial management
PAHO addendum | Creative Commons licences | Protocol or Review
Addendum version
Release date
Explanation of changes from previous version
1
New.
Date | Section | What's new |
|---|---|---|
| Editorial process: titles | Added wording that Methodology Reviews should be handled by the Methodology Review Group only |
| Review proposal forms | Minor corrections to all forms |
| Review proposal forms | Updated forms for Cochrane Reviews of interventions, diagnostic test accuracy, methology, and prognosis studies, and for Cochrane overviews. |
| Review proposal forms | Added form for methodology Cochrane Reviews. | |
| Review proposal forms | Updated forms for Cochrane Reviews of interventions and diagnostic test accuracy studies. Added new form for Cochrane Reviews of prognosis studies. |
| Review proposal forms |
|
| Editorial process: titles | Collated relevant information; added the flowchart (previously linked to |
Date
Section
What's new
| Managing expectations: what does Cochrane expect of authors, and what can authors expect of Cochrane? |
...
| ). |
| General |
Moved the linked flowchart about the editorial process for title registration to Editorial process: titles.
Updated terminology and removed need for Centres to provide support with queries now covered by Cochrane Support team.
...
|
Open access
Date | Section | What's new |
|---|
|
Updated forms for Cochrane Reviews of interventions, diagnostic test accuracy, methology, and prognosis studies, and for Cochrane overviews.
- Renamed "Managing title registration (for Cochrane Review Groups)" to "Review proposal forms". Removed historical note that these forms, developed by the Cochrane Editorial Resources Committee (since disbanded), were previously called Title Registration Forms and were renamed "review proposal forms" in 2013.
- Updated to latest version of forms (August 2019), including revisions for data protection compliance.
- Title change from "Registering titles for new Cochrane Reviews" to two sections "Cochrane Review proposals" (for prospective authors) and "Managing proposals for new Cochrane Reviews".
- Removed text about registering diagnostic test accuracy reviews (out of date).
Open access
...
Date
...
Section
...
What's new
...
...
...
...
...
...
...
Overlapping scope: editorial management
...
Date
...
Section
...
What's new
...
...
- Removed from step 15 following introduction of Cochrane peer review policy: "This will be dependent on the policy of the Host CRG, recognising that some CRGs do not operate an ‘open peer review’ system. The policy of the Host CRG will prevail."
- Changed step 16 to reflect current topics publication process: "The protocol and subsequent review are included in both CRGs’ Topic Lists, which are reflected on the 'Browse' menu in the Cochrane Library." to "CRGs can add the protocol and subsequent review to both CRGs’ topic lists."
...
...
- Combines past 'Avoiding duplication' and 'Overlapping scope' sections.
- Separated into 'Potential overlap with ongoing or published reviews' and 'Potential overlap with new titles'.
| Gold open access | Clarified that PDF version submitted is set out in the licence for publication forms. | |
| Green open access | Clarified that PDF version submitted is set out in the licence for publication forms. |
| Policy overview (open access) | New page for this overview; and added subheading and text for Editorials. |
| Green open access | Clarification of green open access arrangements for Cochrane Reviews and protocols. |
| Open access policy | Green open access policy was updated. |
| Open access policy (first version) | Open-access options were introduced for the Cochrane Database of Systematic Reviews (CDSR). |
Overlapping scope: editorial management
Date | Section | What's new |
|---|---|---|
| Steps to resolve potential overlap with a new title |
|
| General |
|
Peer review: policy
Date | Section | What's new |
|---|---|---|
| Inviting peer reviewers | Redirected link to Peer reviewer conduct from previous reference to a 'Resources for peer reviewers' page. |
| Exceptions to named peer review: special cases | New page added for the post-implementation phase. |
| Type of peer review | Added in text in italic: Note that Consumer peer reviewers are exempt from the named peer review process, and may remain anonymous if they wish to do so. (Consumers still follow the policy for Declarations of potential conflicts of interest for peer reviewers). |
| Peer review policy | Removed "Note: this policy is currently being implemented across all Cochrane groups. It will be implemented fully by all Cochrane groups by January 2019." |
| Type of peer review | Added statement regarding consumer reviewers remaining anonymous. |
| May 2018 | Peer review policy | Organizational peer review policy published. |
Peer review: editorial management
...
Date | Section | What's new |
|---|---|---|
| Inviting peer reviewers | Redirected link to Peer reviewer conduct from previous reference to a 'Resources for peer reviewers' page. | |
| Exceptions to named peer review: special cases | New page added for the post-implementation phase. |
| Type of peer review | Added in text in italic: Note that Consumer peer reviewers are exempt from the named peer review process, and may remain anonymous if they wish to do so. (Consumers still follow the policy for Declarations of potential conflicts of interest for peer reviewers). |
| Peer review policy | Removed "Note: this policy is currently being implemented across all Cochrane groups. It will be implemented fully by all Cochrane groups by January 2019." |
| Type of peer review | Added statement regarding consumer reviewers remaining anonymous. |
| May 2018 | Peer review policy | Organizational peer review policy published. |
Peer review: editorial management
| Updated checklists to include up to date links to Handbook: | |||||
| Inviting peer reviewers | Updated information on what the invitation email to authors should include: "The invitation email should contain the following: The title and abstract and plain language summary of the Cochrane Review (or title only if a protocol);" | ||||
Cleared up an inconsistency between the policy and guidance around Acknowledgement of peer reviewers: The policy states a named peer-review process, but does not state that peer review names need to be made publicly available. The guidance implied that peer reviewer names should be made publicly available unless anonymity was agreed by the EinC. The wording in the guidance has been changed in the following sections to address this inconsistency: | Date | Section | What's new|||
|---|---|---|---|---|---|
| Declarations of potential conflicts of interest for peer reviewers: implementation information | Corrected link to conflict of interest policy | ||||
| Acknowledgement | Corrected Archie search term from "Peer reviewer" to "External referee". | ||||
| Exceptions to named peer review: editorial management | Added "Reason(s) for opting to remain anonymous" to the list of information that needs to be provided to the Editorial & Methods Department. | ||||
| Cochrane TaskExchange: new peer reviewers | New section added. | ||||
| Peer review checklists | Updated guidance for consumer peer reviewers to v3. General updates/revisions to branding/format, contact details, and terminology. Updated peer review checklists to v3. Added data protection statement, updated acknowledgement, links and formatting. |
...